
——Guidelines for the establishment of the China Academy of Advanced Science and Technology

——Guidelines for the establishment of the China Academy of Advanced Science and Technology



One of the important challenges of OA drug therapy is to maintain the effective drug concentration in cartilage tissue to induce the required biological response for a long time. Due to the avascular nature of articular cartilage, systemic administration can lead to insufficient distribution of drugs within the cartilage. Direct intra-articular injection is one of the reasonable methods to increase the bioavailability of drugs in the joint and minimize systemic adverse reactions; Even if administered locally through the joint cavity, the drug is quickly cleared through the synovial capillaries and lymphatic drainage, resulting in a brief retention time of the drug in the joint cavity. The small amount of drugs remaining in the joint cavity is further limited by the dense spatial structure of the extracellular matrix (ECM) of the articular cartilage, which hinders the penetration of drugs into cartilage tissue and further uptake by chondrocytes. Clinical applications require minimizing the frequency of intra-articular injections to reduce the risk of infection. The short half-life of intra-articular drugs and insufficient cartilage diffusion limit the possibility of long-term treatment with OA drugs. Therefore, the ideal drug delivery system for treating OA should be able to fully penetrate the cartilage tissue before being cleared by synovial capillaries and lymphatic vessels, and combine with the components in the cartilage ECM to reduce the physical deformation of cartilage during movement and expel drugs, thereby forming a soft bone specific drug bank for continuous OA treatment.
This study was inspired by the characteristics of chondrocyte matrix interaction and utilized nanotechnology to display natural cell membranes containing various chondrocyte adhesion receptors on the surface of polymer nanoparticles (CM NPs), in order to construct a nanodrug library that mimics chondrocytes and endow the cartilage drug delivery system with enhanced specificity and binding ability. The experimental results showed that CM NPs mimicked chondrocytes in nano scale, inherited the function of facial mask protein on the surface of chondrocytes, and had the homologous targeting of primary chondrocytes mainly through E-cadherin, reticulin mediated endocytosis and megacytosis. CM NPs can specifically adhere to rat and human derived degenerative cartilage ECM and remain in rat cartilage tissue for more than 34 days. In vitro simulated synovial fluid clearance experiments showed that CM NPs (CM NPs Ada) loaded with Wnt signaling pathway inhibitors significantly downregulated the catabolic activity of rat and human cartilage grafts under inflammatory conditions. In rat and beagle OA models, CM-NPs Ada restored pathological gait, subchondral bone remodeling, and slowed down cartilage tissue degeneration in the model animals. The cartilage tissue-specific drug storage platform built by the team is expected to improve the pharmacokinetics of anti-OA drugs and enhance the possibility of long-term treatment with anti-OA drugs.
The research work was supported by the National Natural Science Foundation Basic Science Center Project, the Beijing Natural Science Foundation and the Chinese Academy of Sciences' strategic pilot science and technology project.
Paper link:论文链接
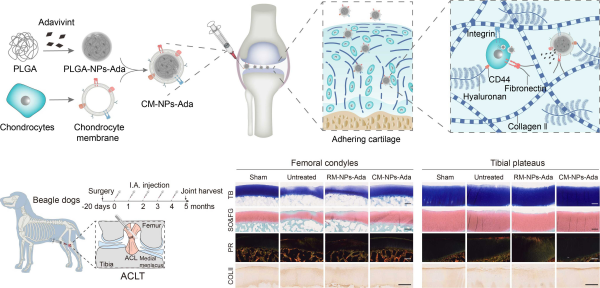
Preparation process, mechanism of action, and efficacy evaluation of biomimetic chondrocyte nanomedicine in large animals
Zhongke Frontier(Xiamen)Science and Technology Research Institute©All rights reserved
Service Customer Service:4006 285 158 Postal Code:361006
Address:Science City Zhongke Building,Huangpu District,Guangzhou City
396 Jiahe Road,Huli District,Xiamen City
Website:http://www.zk-yjy.com