
——Guidelines for the establishment of the China Academy of Advanced Science and Technology

——Guidelines for the establishment of the China Academy of Advanced Science and Technology



Recently, Pan Lifeng's research group published a research paper online in the Journal of the National Academy of Sciences (PNAS) titled "Mechanical insights into the interactions of TAX1BP1 with RB1CC1 and mammalian ATG8 family proteins.". The team used experimental methods such as nuclear magnetic resonance, isothermal calorimetry titration, and spectral shift to investigate the interaction between autophagy receptor protein TAX1BP1 and autophagy initiation ULK complex subunit RB1CC1. For the first time, it was discovered that TAX1BP1 binds to RB1CC1 in a dual site interaction mode. In addition to the known SKICH domain of TAX1BP1 that can bind to the cooked coil domain of RB1CC1, the cooked coil domain of TAX1BP1 can also interact with the C-terminal region of RB1CC1, and the two binding sites of RB1CC1 do not adopt a synergistic mode of action when binding to TAX1BP1. Therefore, the dual site binding mode between RB1CC1 and TAX1BP1 endows RB1CC1 and TAX1BP1 with the ability to form dynamic oligomers. Furthermore, using X-ray single crystal diffraction technology, the team for the first time analyzed the crystal structure of the TAX1BP1SKICH/RB1CC1 cooled coil composite. Based on the team's previous analysis of the SKICH domain of TAX1BP1 combined with the composite structure of NAP1, it was found that the SKICH domain of TAX1BP1 uses different regions and modes of action to bind RB1CC1 and NAP1. Subsequent studies have shown that the cooked coil of RB1CC1 and NAP1 compete to bind to the SKICH domain of TAX1BP1 through allosteric effects. Additionally, the introduction of the FIR motif of NAP1 can mediate the formation of stable RB1CC1/TAX1BP1/NAP1 ternary hexameric complexes.Furthermore, through relevant biochemical experiments, the team confirmed that the binding mode of the RB1CC1/TAX1BP1/NAP1 ternary complex is that NAP1 recruits RB1CC1 and TAX1BP1 simultaneously through its FIR motif and N-terminal coil domain, and the TAX1BP1/NAP1/RB1CC1 ternary complex can further assemble with TBK1 kinase to form a quaternary complex. In addition, this study for the first time analyzed the complex structure of the non classical LIR (CLIR) motif of TAX1BP1 binding to mammalian ATG8 family protein GABARAP, and dissected the molecular mechanism by which TAX1BP1 selectively recognizes ATG8 family proteins through its CLIR motif. The team found that the binding ability of individual TAX1BP1, TAX1BP1/RB1CC1 binary complexes, and TAX1BP1/NAP1/RB1CC1 ternary complexes to ATG8 family proteins was different, and potential mechanisms were explored through relevant biochemical experiments and structural modeling.
This study explores the molecular mechanisms and related protein interaction networks of autophagy receptor protein TAX1BP1 binding to ATG8 family proteins and autophagy initiation ULK complex subunit RB1CC1. It reveals for the first time the unique molecular mechanism of TAX1BP1 binding to ATG8 family proteins and RB1CC1 from a structural perspective, and elucidates the interrelationships between TAX1BP1, RB1CC1, NAP1, and ATG8 family proteins. Research has shown that the autophagy receptor protein TAX1BP1 may first bind to RB1CC1 through a dual site pattern after recognizing ubiquitinated autophagy substrates, thereby recruiting ULK complexes. Among them, the interaction between TAX1BP1 and RB1CC1 not only hinders the binding of the CLIR motif of TAX1BP1 to ATG8 family proteins, but may also lead to oligomerization of ULK complexes and activation of ULK complexes, thereby inducing the production of autophagic precursors in situ. Furthermore, TAX1BP1 recruits NAP1/TBK1 complexes through the NAP1 protein. Among them, the introduction of NAP1 will cause a rearrangement of the interaction between TAX1BP1 and RB1CC1, forming the ULK/TAX1BP1/NAP1/TBK1 super complex, and releasing the CLIR motif of TAX1BP1 to bind to the ATG8 family proteins anchored on the autophagic precursor membrane, promoting the formation of subsequent autophagosomes. This achievement provides new insights into the molecular mechanisms of protein interactions between the TAX1BP1, NAP1, RB1CC1, and ATG8 families from biochemical and structural perspectives, expanding the understanding of the molecular mechanisms of selective autophagy mediated by the autophagy receptor protein TAX1BP1 in the field.
The research work was supported by the National Natural Science Foundation of China, the National Key R&D Program, the Youth Cross Team Project of the Chinese Academy of Sciences, the Shanghai Municipal Science and Technology Commission and the National Key Laboratory for Small Molecule Regulation in Life Process.
Paper link:论文链接
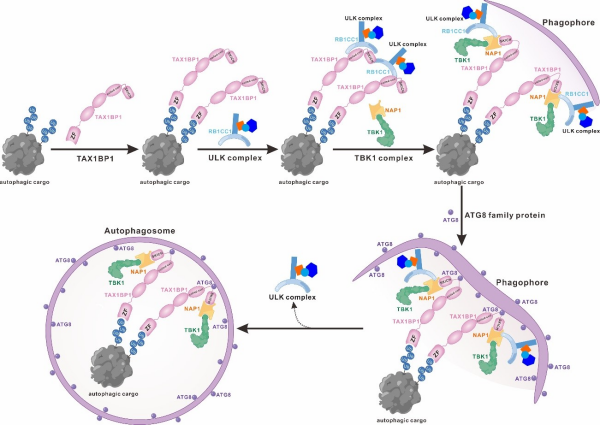
The working pattern diagram of TAX1BP1 recruiting ULK, TBK1 complexes, and ATG8 family proteins during selective autophagy
Zhongke Frontier(Xiamen)Science and Technology Research Institute©All rights reserved
Service Customer Service:4006 285 158 Postal Code:361006
Address:Science City Zhongke Building,Huangpu District,Guangzhou City
396 Jiahe Road,Huli District,Xiamen City
Website:http://www.zk-yjy.com