
——Guidelines for the establishment of the China Academy of Advanced Science and Technology

——Guidelines for the establishment of the China Academy of Advanced Science and Technology



In the outward structure of CHT1 that combines HC-3, HC-3 exhibits a rod-shaped shape and is inserted into the substrate binding pocket from the outside, locking the protein in an outward opening state. In the state of inward choline binding, the intracellular pocket of CHT1 opens inward, and choline remains stable by the tryptophan triad. In an inward substrate free binding state, conformational changes occur in the tryptophan triad pocket, exposing choline to the intracellular solvent side, leading to the release of choline from the substrate binding pocket.
In addition, intracellular short helix IH1 is also an important element that plays a role in the transport process of CHT1. In the outward oriented CHT1 structure, the IH1 helix participates in maintaining conformational stability. When the substrate binds to CHT1, CHT1 transitions from an outward state to an inward state. At this point, the IH1 helix is released, exposing the intracellular pocket of CHT1 and promoting transport. Further experiments have shown that the IH1 helix deficient mutant completely loses substrate transport activity and is unable to transition from an inward to an outward state.
The research work was supported by the scientific and technological innovation 2030- "brain science and brain like research" major project, the national key research and development plan, the National Natural Science Foundation of China and the Chinese Academy of Sciences strategic leading science and technology project.
Paper link
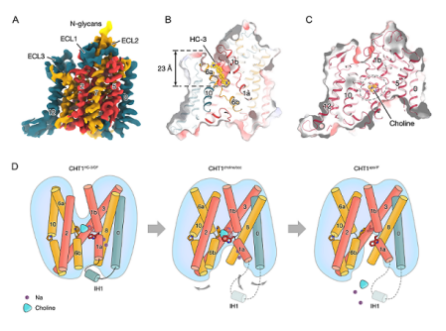
CHT1 binds to different ligands and enters different conformational states, as well as hypothetical substrate transport mechanisms
Zhongke Frontier(Xiamen)Science and Technology Research Institute©All rights reserved
Service Customer Service:4006 285 158 Postal Code:361006
Address:Science City Zhongke Building,Huangpu District,Guangzhou City
396 Jiahe Road,Huli District,Xiamen City
Website:http://www.zk-yjy.com