
——Guidelines for the establishment of the China Academy of Advanced Science and Technology

——Guidelines for the establishment of the China Academy of Advanced Science and Technology



Although the molecular structures of norepinephrine, dopamine, serotonin, and MPP+vary, the structure of VMAT2 complexes that bind to these substrates indicates that they all bind to similar positions in transport proteins. However, the subtle differences in certain functional groups of these substrate molecules result in differences in their interactions and binding patterns with transporters. This reveals how VMAT2 effectively recognizes different substrate molecules. At the same time, histamine binds at a location different from the binding of other substrates. Based on functional experiments, the researchers validated the reliability of this site.
In addition, the study reported the structures of norepinephrine binding to VMAT2 in different conformations, including cytoplasmic and vesicular orientations. During the conformational changes accompanying substrate transport, The N-terminal domain undergoes approximately 30 ° rotation relative to the C-terminal domain, alternately exposing the substrate binding sites on the cytoplasmic or vesicular side, achieving substrate enrichment in the vesicles. Although VMAT2 underwent significant conformational changes, the substrate binding pocket of norepinephrine remained relatively stable during this process, and the interaction with surrounding residues did not undergo significant changes. This clever conformational transformation feature is crucial for the functionality of VMAT2.
Previous studies have reported that the D399 residue may be a key site for substrate binding and protonation, and a hydrogen bonding network has been discovered within the N-terminal domain. This hydrogen bonding network may play a crucial role in coupling protons and promoting the conformational transition of VMAT2. This study confirmed that D33 may be another key protonation site by analyzing the structure of VMAT2 under different pH conditions. Specifically, Protonation of D33 can induce D33 The local conformational changes in the side chains of R189 and Q192 residues disrupted the hydrogen bonding network, thereby promoting the transition of VMAT2 conformation from vesicular orientation to cytoplasmic orientation.
This study analyzed the mechanism by which VMAT2 recognizes monoamine neurotransmitters and neurotoxin molecules with different chemical structures, proposed a molecular mechanism model for conformational changes in VMAT2, and improved the specific mechanism of proton coupled substrate transport. The above achievements provide useful insights into the transport mode of VMAT2, enrich scientists' understanding of the main co transporter superfamily in transporting substrate molecules, and lay the foundation for drug development and optimization.
The research work was supported by the National Natural Science Foundation of China and the Chinese Academy of Sciences strategic leading science and technology project (Category B). The data collection work of cryo electron microscopy has received technical support from the Bioimaging Center of the Protein Science Research Platform of the Institute of Biophysics, the cryo electron microscopy center and soft matter public instrument platform of the Institute of Physics, and the Biological Microscopic Structure Research Platform of the Institute of Modern Agriculture of Peking University. The functional experiment was assisted by the Institute of Biophysics and the Radioisotope Laboratory of the Institute of Microbiology, Chinese Academy of Sciences.
Paper link
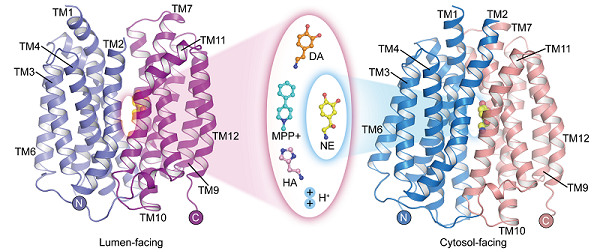
The structure of VMAT2 binding neurotransmitters and neurotoxins
Zhongke Frontier(Xiamen)Science and Technology Research Institute©All rights reserved
Service Customer Service:4006 285 158 Postal Code:361006
Address:Science City Zhongke Building,Huangpu District,Guangzhou City
396 Jiahe Road,Huli District,Xiamen City
Website:http://www.zk-yjy.com